As we roll out our 2023 operational plans, the medical device industry is set to witness some exciting developments and trends. Here are the top five trends to keep an eye on throughout the year:
1 – Artificial Intelligence and Machine Learning
AI and machine learning (ML) are already being used in a variety of medical applications, including image analysis and diagnostic support. We expect to see more widespread adoption of these technologies in the medical device field in the coming years. AI and machine learning can help improve efficiency and accuracy and assist with tasks that would otherwise take longer for humans to complete. We are already seeing this implementation in differential diagnosis by entering the symptoms and having AI eliminate various diseases by coupling the data with the patient’s family history and specific genetic makeup.
monitoring, which is especially advantageous for patients with chronic conditions.
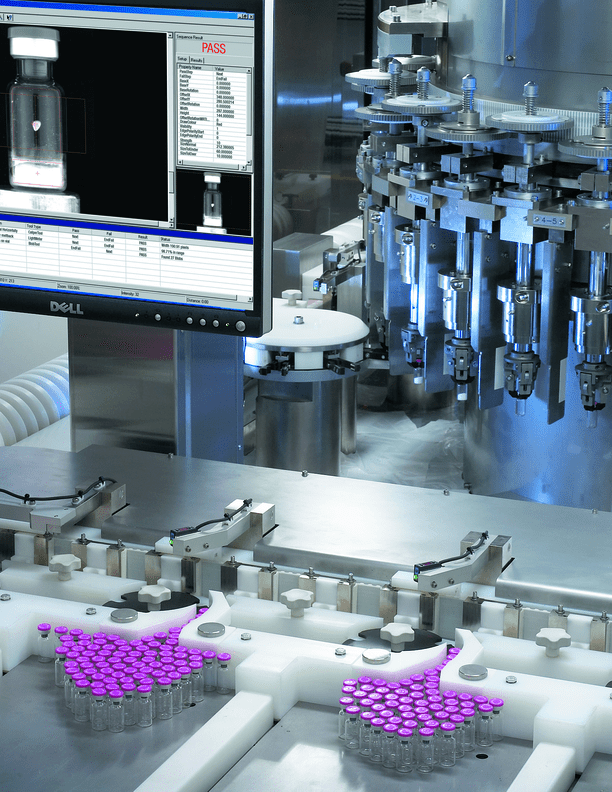
In the field of manufacturing, it is advisable to explore additional prospects to incorporate AI-supported systems in the assembly and testing phases of device production. For example, AI can be leveraged to conduct quality inspections of vials, which involve assessing the stopper’s presence, absence, and position, analyzing the features of each vial’s cap and body. AI can also evaluate the integrity of lyophilized cake or other pharmaceutical contained within a vial. Utilizing AI in these processes allows for faster assessments, which is not feasible with manual methods.
2 – Internet of Medical Things (IoMT)
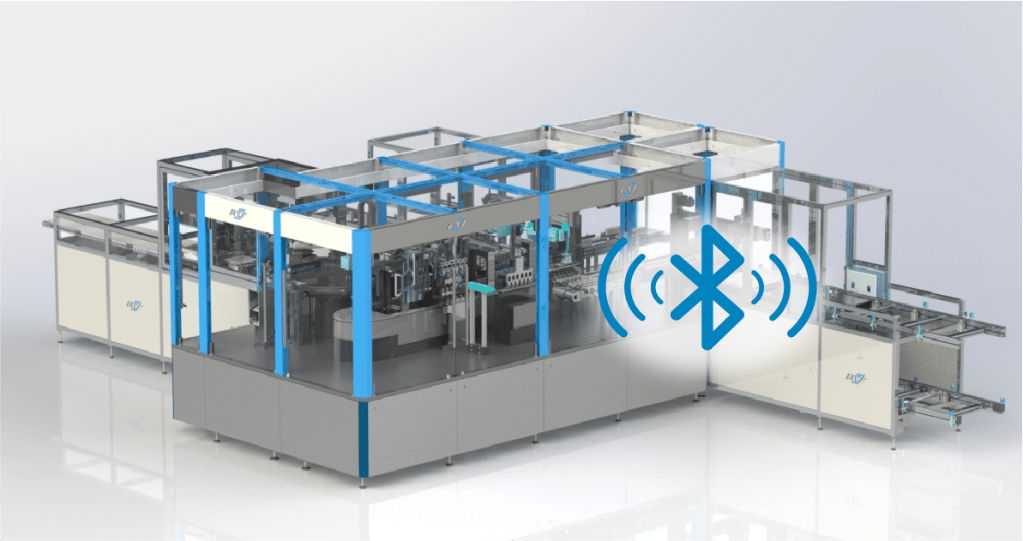
The Internet of Things (IoT) has already transformed many industries, including medicine. The Internet of Medical Things (IoMT) collects and transmits medical data via connected devices. Smart pills, wearable fitness trackers, and remote monitoring systems are examples of such devices. The IoMT has the potential to improve patient care by providing real-time data and enabling remote monitoring, which is especially advantageous for patients with chronic conditions.
In manufacturing, look for automation processes that verify and validate the correct transmission of data and functionality of Bluetooth®-enabled smart medical and diagnostic devices within your automated manufacturing. Test solutions are suitable for smart medical devices like insulin pens, autoinjectors, patch pumps, insulin pumps, inhalers, nasal sprays, etcetera, and smart medical diagnostics like continuous glucose meters, etcetera. The globally connected medical devices market is set to increase five-fold by 2028 over 2020 figures, so a reliable partner with smart credentials is a must for operations planning.
3 – Smart Labels
The medical device sector is utilizing smart labels, a relatively new technology, to increase product safety and traceability. These labels broadcast and store product-related data using RFID (radio-frequency identification) or NFC (near-field communication) technology. The serial number, expiration date, and manufacturer information of the goods can all be accessed by scanning a smart label with a smartphone or other device. Smart labels in the medical device sector can enhance supply chain management and guarantee that items are used properly and safely. They also offer a quick and simple means to track and trace products, which can aid with recall operations and regulatory compliance.
In manufacturing, inline testing through integrated functionality can deliver documentation in a batch report via your PLC-controlled machine system. This kind of reporting is CFR Part 11 compliant with audit trail information integrated within the machine control system.
4 – 3D Printing
3D printing is a rapidly growing field with numerous applications in the medical device industry. Custom prosthetics and implants, as well as complex surgical instruments and other medical devices, can be created using 3D printing. 3D printing of devices also allows for further customization for individual patients, which is out of reach for traditional manufacturing methods that rely on volume for efficiencies at scale.
This technology has the potential to revolutionize the way medical devices are designed and manufactured, making the production of personalized products faster and more cost-effective.
5 – Bioprinting
Bioprinting is a relatively new field that uses the aforementioned 3D printing technology but, in this application, to create living tissues and organs. Although this technology is still in its early stages, it has the potential to transform organ transplantation and drug development. Researchers can test drugs and therapies on human tissue before moving on to clinical trials by creating functional tissues and organs, which saves both time and money. The future of bioprinting is promising with the potential to one day create more complex organs by printing out the specific cells that make up some of our most intricate biochemical systems. Cells, protein, DNA and polymers can be printed in highly complex patterns using microarray printing.
In manufacturing, look for a partner that offers the printing technologies and automation required to print microarrays of diverse content to a variety of substrates from glass, membrane, microwell plates to silicon and more.

These are just a few fascinating changes the medical device industry will likely experience in 2023 and beyond. With the speed at which technology is developing, we can expect that we will witness even more groundbreaking innovations in the years to come.
About ATS Life Sciences Systems
For over 40 years, ATS Life Sciences Systems has worked with the world’s leading manufacturers for product launches, expansions, improvement initiatives, and solving complex manufacturing and process challenges. Whether you need a partner who can supply custom automation systems to anywhere in the world, or whether you need assistance meeting your contract manufacturing needs, ATS has the scale and resources to reduce costs and speed time to market. We look forward to demonstrating our thoroughly tested process in providing best-in-class automation solutions.
A conversation with a reputable supplier will help you understand the feasibility and benefits of automation before you make any decisions. Contact us at lifesciences@atsautomation.com to review your options.














 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube