- SEGMENTS
- AUTOMATION SOLUTIONS
- CAPABILITIES
- SERVICES
- RESOURCES
- About Us
- Brands & Affiliates
- Quality
- Environment
- Locations
- Careers
- Contact
- SEGMENTS
- AUTOMATION SOLUTIONS
- CAPABILITIES
- SERVICES
- RESOURCES

Segments
From contact lenses to mail-order pharmacies, ATS Life Sciences Systems has a long history of providing automated manufacturing solutions for the assembly and handling of a broad range of products.

Automation Solutions
We’ll custom design or help you find the right machines for your Assembly, Material Handling, Conveyance, Manufacturing, Vision Testing, Software (IIoT) needs

SERVICES
Our ability to assist you with your project begins with understanding your product and processes. Whatever the stage of your product’s life cycle—product design, product iteration, clinical trials, or full commercial production—ATS Life Sciences Systems can complement your staff with CGMP-experienced consultants, engineers, and skilled trades and service people

Resources
A deeper dive into information and details about LSS solutions, from the experts who work on them every day.
Sign up for Life Sciences News and Updates
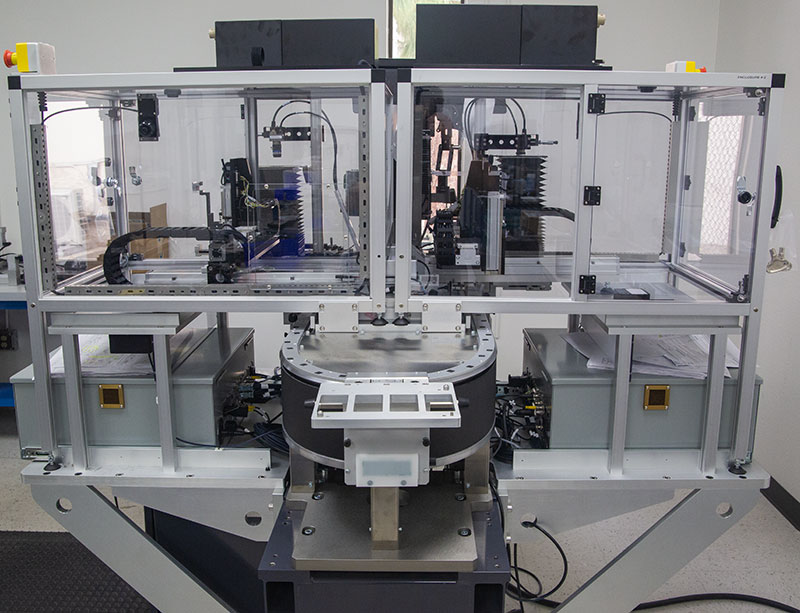
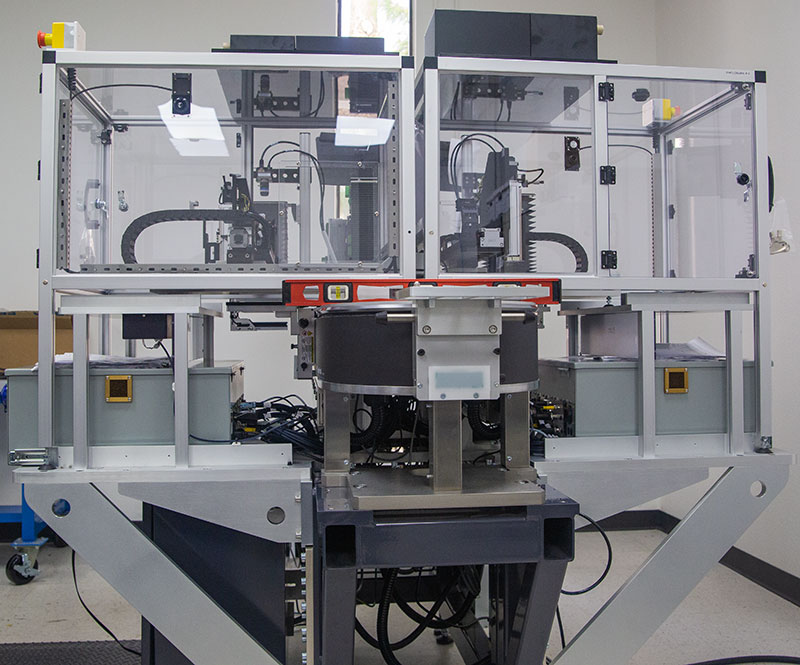
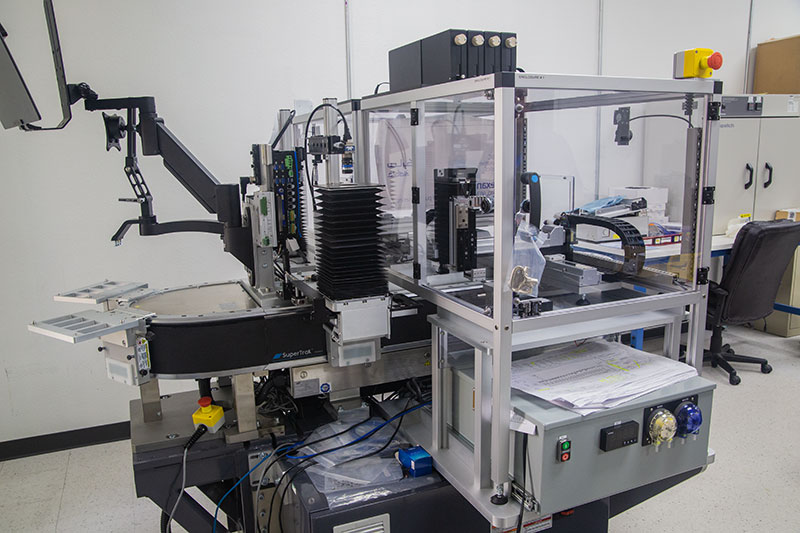

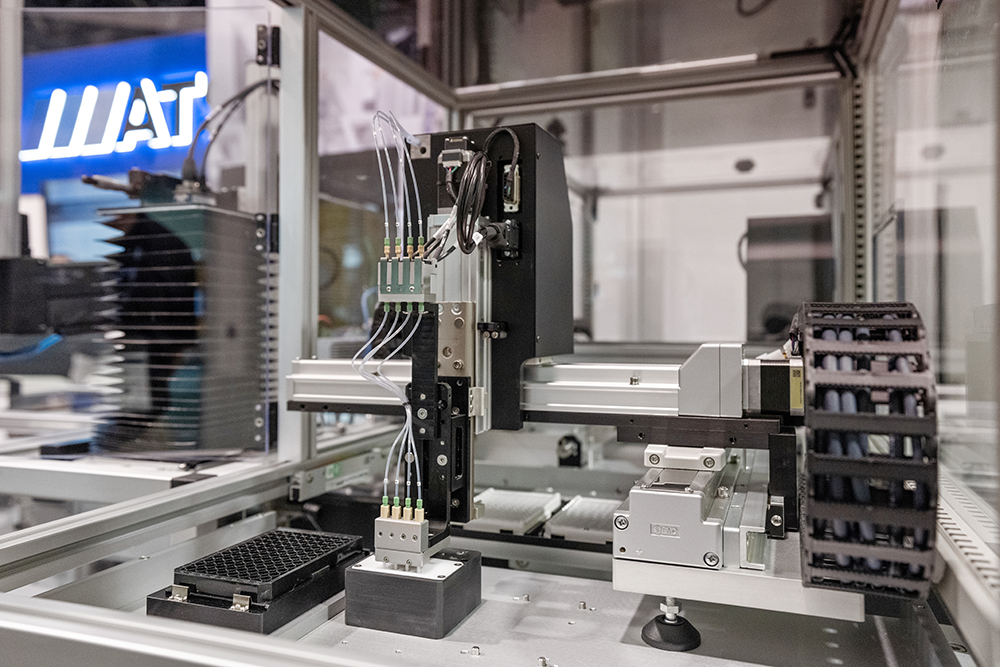







MODULIS™ Dispensing for Miniaturized Diagnostic Manufacturing
PoC and Consumer Diagnostics
The ATS Life Sciences Group delivers fast-to-market and seamless integration to achieve manufacturing at scale. Modular, configurable dispense and smart conveyance platform for low and high-volume point of care manufacturing and diagnostic device assembly.












MODULIS™ Dispensing for Miniaturized Diagnostic Manufacturing
PoC and Consumer Diagnostics
✕
OUTPUT LEVELS
Additional information
Diagram
Clinical, LV or HV Automation Platform
(Estimated order to FAT complete lead time)
- Clinical (~2 ppm)
Does not include SuperTrakTM CONVEYANCE
< 6 months
- Low volume (up to ~15 ppm)
Includes SuperTrakTM CONVEYANCE
< 10 months
- High volume (> ~ 15 ppm)
Includes SuperTrakTM CONVEYANCE
< 12 months
Features
- Expandable conveyor and workstation configuration to meet unique customer throughput requirements
- Equipped with:
- IlluminateTM Manufacturing Intelligence
- ATS CortexTM Vision System
- Variable throughput microfluidic manufacturing, biosensor, biochip, lab on a chip (LOAC), diagnostic, microarray
- PLC Master Control
- Integrated device processing (drying, cooling, humidity control)
Recommended for
- Diagnostic Cartridges and Wearables
- Single or Low Plex Assays
- Quantitative and Qualitative Testing
- Amino and Genomic Assays
- Clinical analyzers to detect presence of infectious disease (eg. POC lateral flow tests)
- Consumer Genomics
Learn More About Microfluidic Dispensing
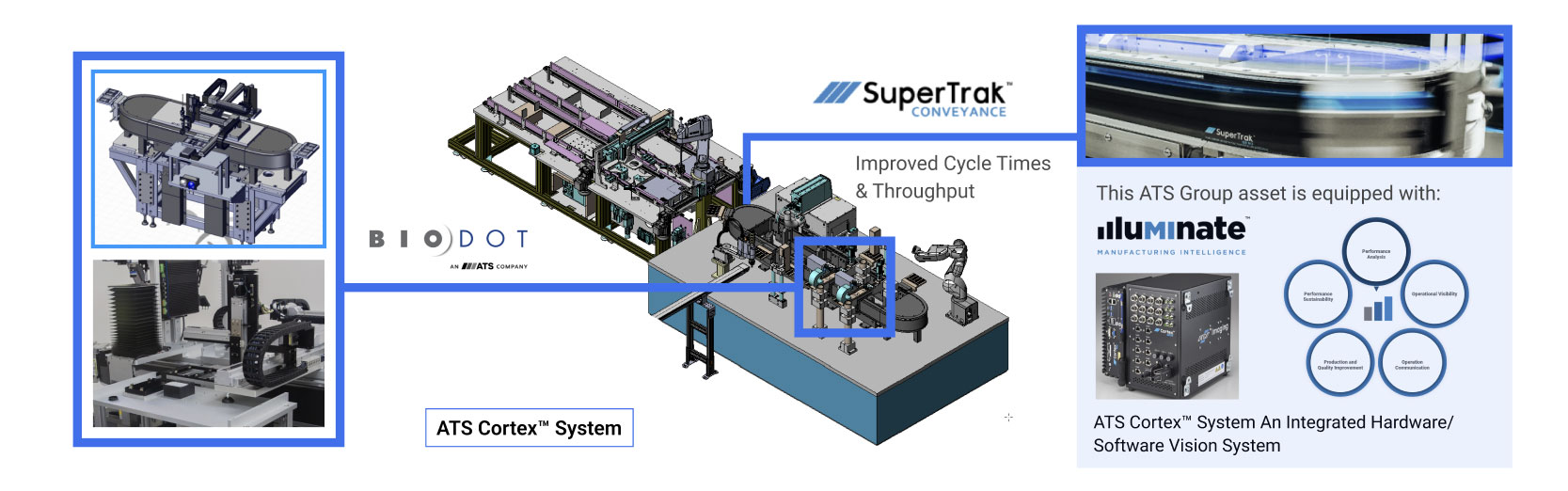
BioDot is a specialist in ultra-low volume dispensing; picoliter to microliter discrete product dispensing, complemented by enabling technologies, Application engineering, product validation, and DFA/DFM. Experience supporting customers from research to pilot production.
The MODULISTM solution offers vision-guided part targeting, at 2 nanoliter to 4 microliter discrete droplet volume with up to 24 dispense channels.


Learn More About Smart Conveyance
The SuperTrak CONVEYANCE™ is a fully programmable asynchronous linear motion technology with independent and parallel dispensing workstations. Integrated parent PLC cell control, integrated smart vision, modular and configured system footprint.
Index shuttles quickly to eliminate the number of tooling parts required. Turnkey solutions are fully supported over the life of the asset with support for IQ / OQ. MODULIS, equipped with SuperTrak CONVEYANCE™ is a modular, integrated solution that transitions from clinical to high-volume manufacturing.
Large diagnostic organizations and start-ups alike are focused on three key areas:
Reducing time to result,
Reducing sample volume, and
Increasing the quality (and quantity) of the underlying patient data.
Smaller, more complex devices with 10s to 1000s of individual capture chemistries are currently moving through the development cycle, and the most advanced groups are now bringing their solutions to market.
Download our whitepaper for a more in-depth look at the opportunities in the diagnostic landscape.
Resources
Infographic
The Road to Commercialization
MODULIS is a modular solution for clinical and high-volume manufacturing. It shrinks your automated micro-dispensing platform build schedule from order to factory acceptance.

Blog
Consumer Diagnostic Device Manufacturing
The race is on to develop and commercialize new novel biochips/biosensors for a broad spectrum of health markers. Getting to market first is the challenge.

FAQ
Frequently Asked Questions
Customers want to know how we developed this pre-configured platform, and what its capabilities are from the lab to high-volume production.
Customization, reduced start-up time, and integration expertise coupled with global reach and local support
We solve your manufacturing challenges with proven technology to get you to market fast. Tailor the modules to meet demand as you move from clinical production to commercialization.
GET IN TOUCH
Briefly tell us about your automation needs and we’ll get back to you.
👋 Looking for something? I'm Gears and am happy to help.



 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube