Choosing the Right Partner to Transform from Parts/Day to Parts/Minute Output – Centered on Ultra-Low Volume Dispensing in POC Diagnostic Manufacturing
Introduction
Ultra-low volume dispensing for this discussion is the ability to dispense precise single droplets that are basically invisible to the human eye and defined in units of pico or nano liter volume. We care about this because the dispensing process is core to the manufacturing of miniaturized devices including microfluidics, biosensors, biochips, microarrays, and implantable wearables.
Standalone dispensing equipment to meet volume and precision requirements is readily available for manually operated, low output (meaning less than two parts per minute) solutions in research and development or clinical environments. What isn’t commonplace is to find a technology partner to help diagnostic MedTech companies scale their manufacturing output, integrate up- and downstream manufacturing processes adjacent to dispensing, and transform their capacity to meet market demand sooner than later.
The Challenge
The global demand for POC diagnostics, whether measuring blood glucose or detecting the presence of an infection, is in growth mode. As a result, there is a critical need to find the right technology partner that has made the investment in designing and installing micro-dispensing technology specifically implemented in the manufacturing of medical and consumer diagnostic devices. If you are responsible for the operation of highly multiplex/microarray device manufacturing and validation that requires ultra-low volume dispensing, you are probably faced with the challenge to navigate the roadmap to ramp from low volume output to higher output all while in the race to deliver sellable product to the market.
In the event that you need to search for a technology partner to join you on your capacity expansion journey, you will either find an organization that is an expert at dispensing and builds dispensing solutions as a stand-alone unit, or you will find an automation integrator that procures a third-party dispensing solution, in which case you hope for the best that they have the subject matter experts and experience to integrate the dispensing and inspection processes within your automated line.
From Good to Great
1 – The journey from parts per day to parts per minute in commercializing a device includes design for manufacturability, and design for automation, assuming the device design is close to being frozen. Finding a dispensing OEM or an integrator to set up a manual test bench or clinical semi-automatic solution is relatively straightforward and that is good. However, it’s probably not great when ramping up to higher output because now the need is for a fully automated and configured solution that expands the level of technical risk for the obvious fact that output is increasing.
Additionally, the risk is compounded because the current priority for MedTech companies is shrinking the time to market to deliver sellable product, especially in the diagnostic segment, because the competition in the development of new novel POC diagnostics is fierce. So, comparing organizations that help achieve the time to market objective and can deliver an end-to-end high output manufacturing system is critical.
The good news is there are three types of organizations readily available: (1) the system integrator that integrates third party equipment but doesn’t own the IP for the process equipment such as dispensing, (2) the dispensing equipment builder that doesn’t integrate automated systems, or (3) an entity that offers automated dispensing for higher outputs but doesn’t automate the adjacent up- and downstream processes to the dispensing.
Moving from good to great in evaluating a technology partner for a parts/min need requires a new option.
| Example Comparison of Partner Requirements to Transform Low Output to High Output Device Manufacturing | |
| Parts/day | Parts/minute |
| Support – access to process SMEs specific to dispensing | Support – multiple cross-functional SMEs to support end-to-end Mfg. process |
| Standalone ‘standard’ equipment | Standard and customized for the output required |
| Little to no connected/integrated processes | Manufacturing processes connected by material handling (i.e. conveyance) |
| Potential for light automation | Controls intensive; part and batch traceability |
| Technical risk is low – no sellable product | Fully automated processes including component feeding, dispensing, assembly, inspection |
| Minimal validation | Technical risk is relative to device complexity and rate |
| Light service | Asset life cycle management program to sustain equipment performance |
| Fully validated manufacturing line including IQ, OQ, PQ | |
The ATS Life Sciences Group falls into the new category as we combine all the elements to enable low output to high output via custom integration, smart conveyance in the SuperTrakTM linear mover, BioDot dispensing technology, ATS SmartVisionTM, and IlluminateTM Manufacturing Intelligence.
The MODULISTM platform is a proven platform that is in the field manufacturing diagnostic devices now. The MODULIS system is a modular and seamless platform that utilizes one control system to manage all manufacturing processes and the smart conveyance platform. This is unique technology that is unmatched in the ability to acquire production data for managing machine performance as well ease of use, setup, and maintenance. For example, the BioDot dispensing system has been converted from a PC-based control to a PLC control that also controls all the other motion and associated process stations, as well as the machine vision for inspection.
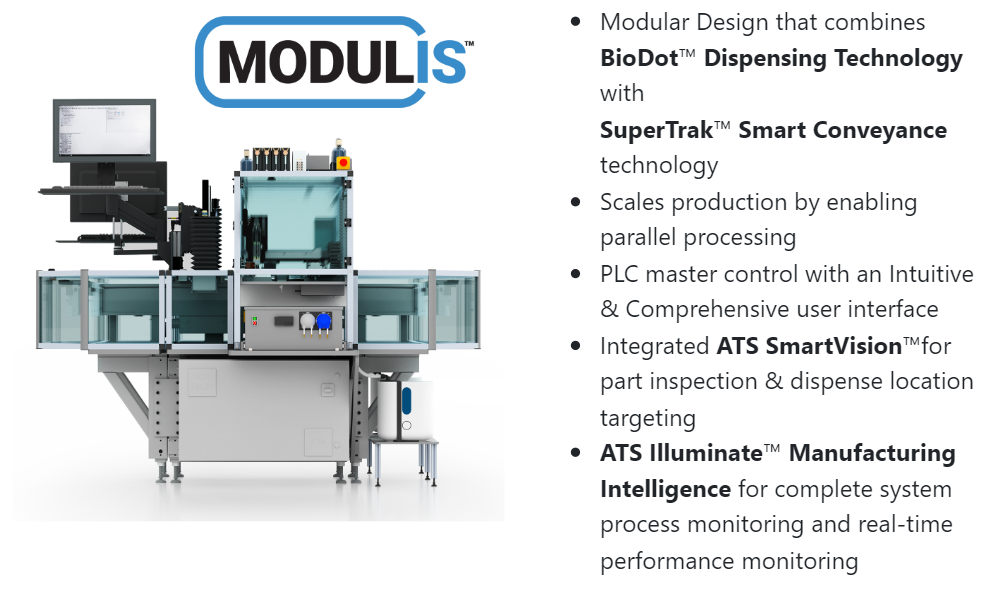
2 – The next partner evaluation category is to compare the evidence that demonstrates a project execution plan enabling your time-to-market objectives based on your starting point. Some organizations are good at providing a standard solution that is developed around their specifications) that may or may not be rigid or allow for much customization, as every device and application is unique). Additionally, the standard offering should be vetted in terms of modularity to scale output. What great looks like is the ability to incorporate solutions that offer the following criteria:
- Ability to automate existing dispensing assets as well as add additional dispensing stations, inspection stations, and up/down stream capability to incrementally add capacity as needed;
- Ability to integrate from a control’s perspective all the process stations into one main PLC control which enables ease of production and performance monitoring
- Ability to modify footprint slightly as the need for capacity is needed; this is the smart conveyance connecting all the assets;
- Ability to do retrofits for future expansion because companies that build standard solutions are generally not excited about retrofits and the investment it takes to execute well.
The image to the right captures the intent of what great looks like. Expanding capacity from the R&D and clinical phase to low and high output using the same platform requires using technology that is modular and scalable. This minimizes capex expense throughout the commercialization journey and allows you to expand incrementally.
A real example is noted below in comparing good to great in this principle of a seamless and modular turnkey platform that can be customized. Examine the output difference as well as the cycle time for what would be a standard offering versus a modular, seamless platform designed for scale.


GOOD
AD 6020
12 Channels
Total Time: 56 minutes for 20 plates (0.3 PPM)
With Inspection: 2 hours for 20 plates (0.15 PPM)

GREAT
MODULIS
8 Channels
7 Stations
2.3 PPM (with inspection)
3 – The third criterion in comparing partner capability as good or great is their ability to support the roadmap from research and development through high output commercial production. Are they good at one aspect of your roadmap, but not the other? Or are they great in offering an end-to-end approach including the life cycle management of your assets over the course of their useful life?
ATS brings several technologies that enable life science companies to manage risk early in the development cycle as well as make informed decisions about their approach to high output manufacturing. These tools include the ability to deliver digital twin models for simultaneous engineering of the MODULIS platform including dispensing and all value-added stations. Additionally, the SuperTrak TrakMasterTM simulation tool is available to optimize right-sized output as well as buffers in a high output workflow. Since ATS is the original equipment manufacturer of the smart conveyance, the dispensing technology, and the vision inspection technology, ATS controls the quality and the life cycle performance better than any integrator that must procure these assets from outside sources. That is better than just being good, it’s great.
Challenge us
There were three areas to compare a good offering and a great offering in terms of automated, high output ultra-low dispensing. This isn’t a niche market anymore. Millions of patients buy continuous glucose monitoring devices, as well as rely on reliable diagnostic cartridges with their heath diagnosis. There is a clear differentiation that the ATS Life Sciences Group brings to this space in making significant investment in the invention of dispensing, inspection, simulation tools, and smart conveyance, but ATS stands with our life science device and diagnostic partners for the duration of their journey from research and development to the finality of retiring a long-performing asset. The devil is in the details in terms of how the various assets are integrated together, can be configured for future products or process changes, and ease of use. There is a difference in reliability and quality. Like automobiles, we’re asking you the reader to look under the hood to select the right partner.
How to contact us
It costs nothing to investigate how the ATS LS Group can help you achieve your commercialization objectives. Please connect with us at lifesciences@atsautomation.com













 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube