- SEGMENTS
- AUTOMATION SOLUTIONS
- CAPABILITIES
- SERVICES
- RESOURCES
- About Us
- Brands & Affiliates
- Quality
- Environment
- Locations
- Careers
- Contact
- SEGMENTS
- AUTOMATION SOLUTIONS
- CAPABILITIES
- SERVICES
- RESOURCES

Segments
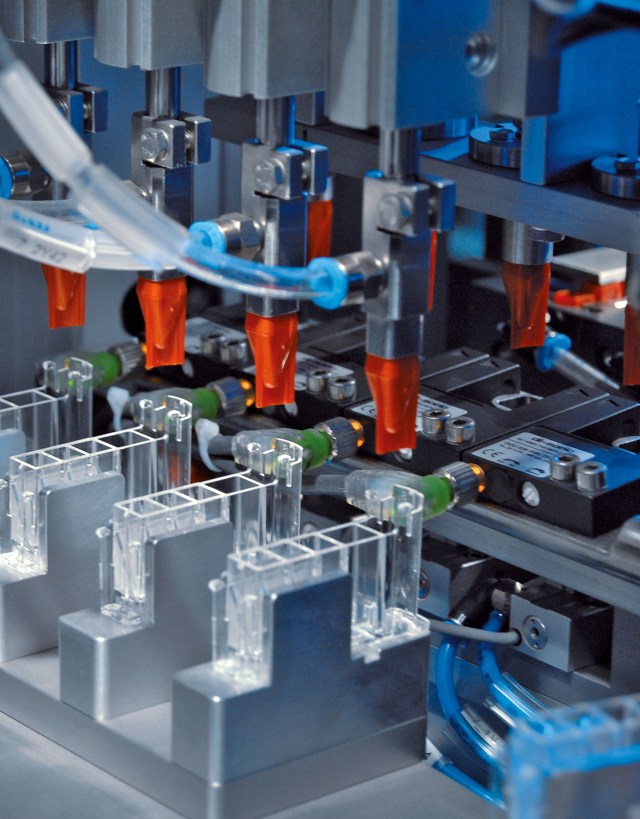
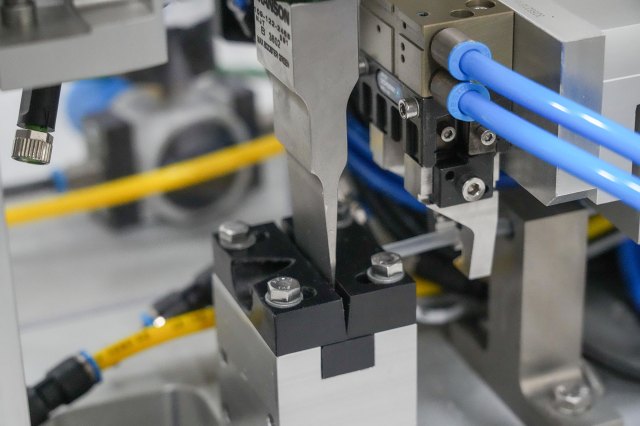
From contact lenses to mail-order pharmacies, ATS Life Sciences Systems has a long history of providing automated manufacturing solutions for the assembly and handling of a broad range of products.

Automation Solutions
We’ll custom design or help you find the right machines for your Assembly, Material Handling, Conveyance, Manufacturing, Vision Testing, Software (IIoT) needs

SERVICES
Our ability to assist you with your project begins with understanding your product and processes. Whatever the stage of your product’s life cycle—product design, product iteration, clinical trials, or full commercial production—ATS Life Sciences Systems can complement your staff with CGMP-experienced consultants, engineers, and skilled trades and service people

Resources
A deeper dive into information and details about LSS solutions, from the experts who work on them every day.
Sign up for Life Sciences News and Updates

Case Studies
Our customer, a global pharma company, is a producer of a cancer drug containing both a liquid and a powder that is delivered via syringe. Their existing assembly process was outdated, required staff in full PPE garb in cleanrooms, and was not Class A. They wanted an automated aseptic filling, handling and packaging line that included containment of APIs in the form of a liquid and a cytotoxic powder, and that was capable of increasing production capacity. Initially the request was for two separate machines: one that could produce a syringe line and a second that would handle vials. After consultation with the experts in the ATS LS Group, we were able to present a hybrid concept, merging the two processes and delivering a single end-to-end solution capable of changing formats from one to the other, quickly and efficiently. Despite greater complexity, the revamped proposal was less costly and mitigated risk to both the product and to operators.
Our customer, a global pharma company, is a producer of a cancer drug containing both a liquid and a powder that is delivered via syringe. Their existing assembly process was outdated, required staff in full PPE garb in cleanrooms, and was not Class A. They wanted an automated aseptic filling, handling and packaging line that included containment of APIs in the form of a liquid and a cytotoxic powder, and that was capable of increasing production capacity. Initially the request was for two separate machines: one that could produce a syringe line and a second that would handle vials. After consultation with the experts in the ATS LS Group, we were able to present a hybrid concept, merging the two processes and delivering a single end-to-end solution capable of changing formats from one to the other, quickly and efficiently. Despite greater complexity, the revamped proposal was less costly and mitigated risk to both the product and to operators.
Quantifying, understanding and verifying product quality is a critical step in any manufacturing process. Moving from a manual inspection to an automated inspection provides greater insight into the quality of the product and process by creating measurable baselines and enabling statistical process controls. This case study provides an overview and takeaways from an ATS project that involved designed flexibility, close collaboration with the customer on their evolving needs, and advanced technologies including Artificial Intelligence-based (AI) inspection.
GET IN TOUCH Briefly tell us about your automation needs and we’ll get back to you. More Case Studies
Same Speed, 90% Less Tooling and TWO Different Autoinjectors on One Machine






 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube