Smaller, Faster, and Cheaper
The pharmaceutical industry, the broader life science industry, and academic research have always led the move towards highly multiplexed data collection in order to answer more questions per day, per hour, and per minute. The singular goal has always been to continually drive and develop technology that enables the absolute highest number of data points per run. In the late 90s and early 2000s, 96-well SBS formatted plates quickly moved to 384 wells, then 1536, and then 3456. Today, we see groups developing on plates (or novel devices) with over 100,000 targets per plate or chip.
Now we see that technologies like digital PCR, single-cell multiomics (genomics and proteomics) and spatial genomics are both informing and shaping the future of diagnostics. Early gene expression microarrays and high throughput drug screening techniques laid the foundation for over 20 years of continual evolution that ultimately resulted in where life science is today: low volume/high throughput sequencing assays, high content cell-based assays, complex protein microarrays… all supported by analytics and AI models to process the huge data sets that are now being created in every lab around the world.
So how (and why) did the diagnostics industry leverage these discoveries in pharma, academia and the broader life science industry?
To answer the question, it’s important to remember the diagnostics industry has been, and will always be, driven by its basic tenets:
- Drive down patient sample volumes to simplify collection
- Reduce time to result
- Expand the test menu to capture all relevant biomarkers or to add more diseases per device
- Reduce cost
When we start to consider these tenets, it’s obvious why the work done in basic discovery has its fingerprints all over the current state in diagnostics. Implantable/wearable sensors for diabetes monitoring, cartridge-based molecular diagnostics, highly-multiplexed and microarray-based ELISA, and high-throughput mass spec have all leveraged previous innovations to solve their problems.
How do we reduce time to result?
Early lab-on-a-chip (LOAC) and microfluidic technologies were designed to address the problems associated with multi-step assay protocols. Cumbersome amplification steps were designed into the assay device and now we see these elements in existing and emerging diagnostic devices. Laboratory protocols that previously took days are now performed in minutes on a single device. More complicated but smaller devices are now common and the trend that will continue to drive the industry. The implication is that production methods now must manage more precise assembly with respect to parts and reagents.

How do we reduce patient sample volumes?

Smaller SBS formats (the move from 96 well plates to highly dense plates and microarrays) drove the need for ultra-low volume dispensing technologies. Non-contact microliter, nanoliter, and picoliter dispensing systems enabled the move to smaller wells and devices and are now deployed across many life science and diagnostic applications. Smaller wells equate to smaller total reaction volumes. This ultimately saves costs in life science reaction and saves patient volumes in diagnostics.
How do we expand our ability to test multiple biomarkers/analytes per device?
The first genomic and gene expression arrays were looking to solve the same problem. We commonly see 30,000 or more individual oligonucleotides on a single microscope slide (25mm by 75mm). Diagnostic formats don’t require this level of extreme multiplexing, however the density achieved by a DNA microarray are commonly leveraged to analyze 10-100 chemistries with a similar density. This means that huge numbers of biomarkers can be added to novel diagnostics without forcing unrealistic changes to device size or needed patient sample volumes.
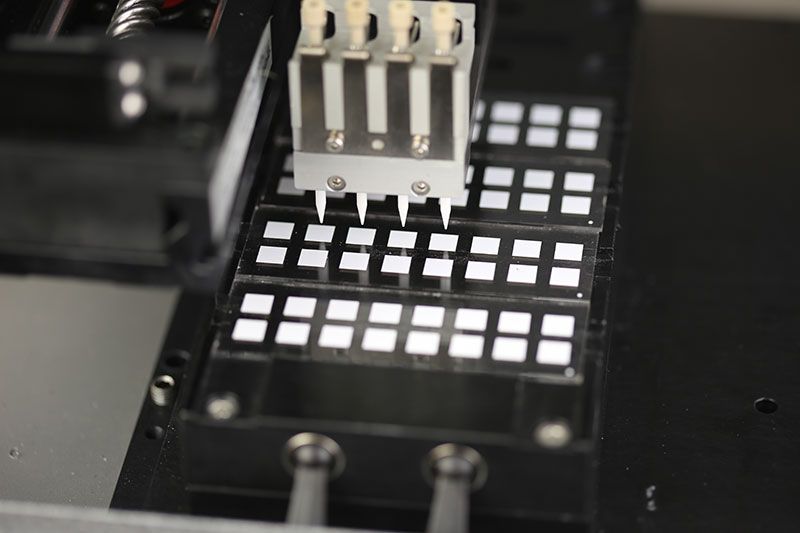
So how do we achieve this? How do we produce highly complex devices and assays that require nanoliter volumes and many different chemistries per device?
We need a nanoliter dispensing technology that is designed for production and we need a conveyor system that can move devices with a precision that matches the sizes and dimensions that small devices require. The number of chemistries on the device significantly complicate the manufacturing process and, while dedicated liquid handling workstations can be deployed to meet any ultimate throughput requirement, the solution may not support cost of manufacturing metrics. An integrated conveyor solution (adding multiple dispensing workstations on a single conveyor) increases system throughput, reduces operator needs, and reduces the total manufacturing footprint.
The ATS Life Sciences Group has deployed its people and its technologies to address this need; MODULISTM is the solution. High-speed printing systems and conveyor segments have been designed to adapt to any device and any throughput. Configurable, modular, and scalable segments allow us to quickly deliver semi-custom solutions and reduce time to market.











 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube