25pL to 4.0uL dispensed discrete drop volume now capable in high output automation platforms
According to a 2023 report, Global News Wire is reporting a 12.7% CAGR growth and greater than $80B USD in revenue by 2028 in Point of Care (POC) diagnostics. This tremendous growth is fueled by continuous glucose monitoring wearables, as well as the demand that was kick started with the advent of a myriad of at-home COVID-19 PCR and rapid tests. The race is on now for consumer and MedTech companies to develop and commercialize new novel biochips/biosensors that can provide a broad spectrum of quantitative and qualitative health markers from detecting presence of an infectious disease to measuring physical preparedness for a competitive athlete. Additionally, we want these tests readily available in our local pharmacy without a doctor’s prescription or by clicking ‘Buy’ on an e-commerce website. We want multiple data points from our diagnostics such as presence of flu and/or Covid-19. We also want to wear these sensors for the ability to receive real-time feedback.
At the heart of a biosensor/biochip that provides the resulting health marker data to the consumer or patient is the diagnostic device microfluidic path that enables the user’s specimen to interact with the assay which contains the unique reagent chemistry. Medtech and consumer organizations are in a development frenzy following the COVID-19 pandemic to develop specialized reagents in the form of a diagnostic device they can bring to market faster than anyone else.
The challenge to time-to-market with a sellable product is the leap from R&D onto a clinical phase, and then to commercialized manufacturing. In response to accelerating the time-to-market, the ATS LS Group has combined and integrated multiple device manufacturing technologies specifically targeted at discrete droplet dispensing and smart conveyance that achieves low and high output manufacturing.
People want rapid quantitative health data that is easily accessible

Whoop, Fitbit, and Apple Watch are examples of on-body wearables that provide health marker dashboards from sleep to exercise performance. When you feel sick, you go to your health care provider and hope to have a health panel telling you about your condition(s) as fast as possible. Pharma companies including Roche, Quidel, Abbott, and Hologic provide the diagnostic tests and readers that provide the patient on-the-spot feedback, or shortly thereafter.
Winning the time-to-market race for Pharma organizations is ultra-critical because as consumers we want easy access to tools that let us know the first signs of any alterations in certain health markers, and we want to know leading indicators long before we present physical symptoms. Detecting these changes early allows proactive action and, if necessary, to get treatment or change our lifestyle and avoid more serious consequences.
Where the time-to-market battle is won
It’s one thing to invent new chemistries that can detect a particular infectious disease, and another to have the expertise, resources, and know-how to develop the sellable product roadmap and thus capitalize on the short opportunity window to launch to market before someone else does. The ATS LS Group recognizes the roadmap gap especially in device design for manufacturing and automation activities. Achieving a sellable device design is way beyond chemistry. We’re talking about a model for competitive cost of goods, competitive cost of manufacturing, and manufacturing processes including assembly, dispensing, and inspection processes. This is where the time-to-market battle is won.
Another market driver not yet mentioned in the POC diagnostic space is the market demand for miniaturization of on-body wearables. Miniaturization is driving more complexity in the device development roadmap, having to utilize micro molding techniques and the feeding of smaller components in a production assembly system. The ATS LS Group understands and works with manufacturers in this space starting with a risk-based approach to design for manufacturability and design for automation (DFM and DFA).
The ATS LS Group consists of a consortium of life science technology companies combining forces under one organizational structure with a common goal to help Pharma companies bring their products to market first and fast. In the context of POC diagnostics, BioDot and ATS Life Sciences Systems combined ultra-low dispensing technology that delivers discrete nano and pico liter droplet size with the SuperTrak Gen3TM smart conveyance asynchronous platform. These technologies are fully integrated to deliver an automated platform that is modular and especially scalable for accelerating manufacturing output from pieces per week to pieces per minute.
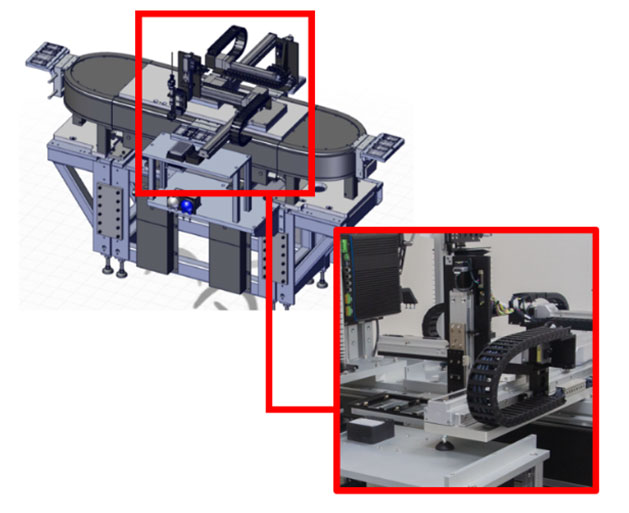
The illustration above describes the integrated solution named MODULISTM. The differentiated features and benefits of MODULIS compared to any commercial nano/pico liter dispensing solution include:
- Coordinated motion between the smart conveyance and the dispensing X, Y, and Z axis linear guides driving the dispense heads into position
- Integrated parent PLC cell control
- Integrated ATS CortexTM Vision inspection platform
- Modular and configurable system length and outputs of 5 ppm up to 100+ ppm
- Fully integrated with the full manufacturing workflow including assembly, and optional packaging
- IlluminateTM Manufacturing Intelligence onboard package for comprehensive production monitoring and optimization.
The ATS LS Group is also committed to supporting IQ and OQ validation, as well as a comprehensive asset life cycle management strategy.
This MODULIS platform is available in the ATS LS Group Innovation Center for proof of principles that help manufacturers transform research concepts to reality. Additionally, MODULIS supports simulation tools using SuperTrak TrakMasterTM software to optimize design for automation activities. The illustration on the right shows the simulation tool developed specifically for MODULIS.
The ATS LS Group collectively brings more than 40 years of experience in delivering automated solutions to Medtech and Pharma manufacturers. Our dispense solutions are prevalent in research labs today and we’re ready to engage in a pre-automation initiative with your team. The ATS LS Group is a proven industry leader in DFA/DFM as well as delivering on-time turnkey automation for the assembly of POC diagnostic devices. Our pre-automation engagement includes URS development, Functional Requirement Specification documents, simulation, and a digital twin approach that supports simultaneous engineering activities. The ATS LS Group always takes a risk-based approach in helping manufacturers achieve their commercialization objectives. As a result, we have been fortunate to leverage a broad set of experiences in developing and implementing research to sellable product roadmap.
Data, data, and more data
There is no doubt that patient and consumer health care is dynamically changing and moving at an accelerated rate for easy, accurate, rapid, and miniature wearables and diagnostic devices. From Ancestory.com and 23andMe to the Abbott ID Now COVID-19 PCR test, we’re just at the precipice of at-home point-of-care diagnostic testing. The ATS LS Group has pulled off miracles during the height of the COVID-19 pandemic to help manufacturers achieve incomprehensible time-to-market objectives. There were numerous lessons learned from a manufacturing perspective to produce high-output diagnostic devices that, in collaboration with some diagnostic device manufacturers, the MODULIS platform was borne. The MODULIS platform is being utilized today for POC diagnostic device manufacturing at high output.
The MODULIS platform supports compressing the equipment build schedule by leveraging standard platform designs reducing the design phase of a project, as well as reducing the procurement and assembly and test phases in a platform build.
The unique ultra-low dispensing solutions that BioDot invented are a leading-edge dispensing solution critical to the development of diagnostic multiplex assays.
It costs nothing to investigate how the ATS LS Group can help you achieve your commercialization objectives. Please connect with us at https://atslifesciencesgroup.com/.










 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube