Automated Plasma Isolation: 500 Patient Samples Separated, Fractionated, and Isolated Per Hour
In this post we will walk through the ATS approach to clinical laboratory automation, specifically as it relates to sample receipt and pre-analytic testing for plasma isolation, and how our solution provides quality samples to ensure critical test results are as accurate as possible.
Every year billions of blood tests are run in labs across the world. These samples move from where they’re drawn to an analysis lab, making many stops such as sorting, accessioning, and analysis before arriving at the results of the test.
Plasma isolation is one of these critical stops, and the quality of this process can directly affect the quality of the test results. So, when developing a system to automate this process, careful consideration must be given to ensure it is robust, accurate, and effective at producing the high-quality plasma that blood tests require.
An ATS laboratory automation solution, utilizing the SuperTrak CONVEYANCETM platform in conjunction with BioDot aliquoting channels, can accurately detect plasma levels and isolate the plasma from the remaining buffy coat and red blood cells with patient blood samples.
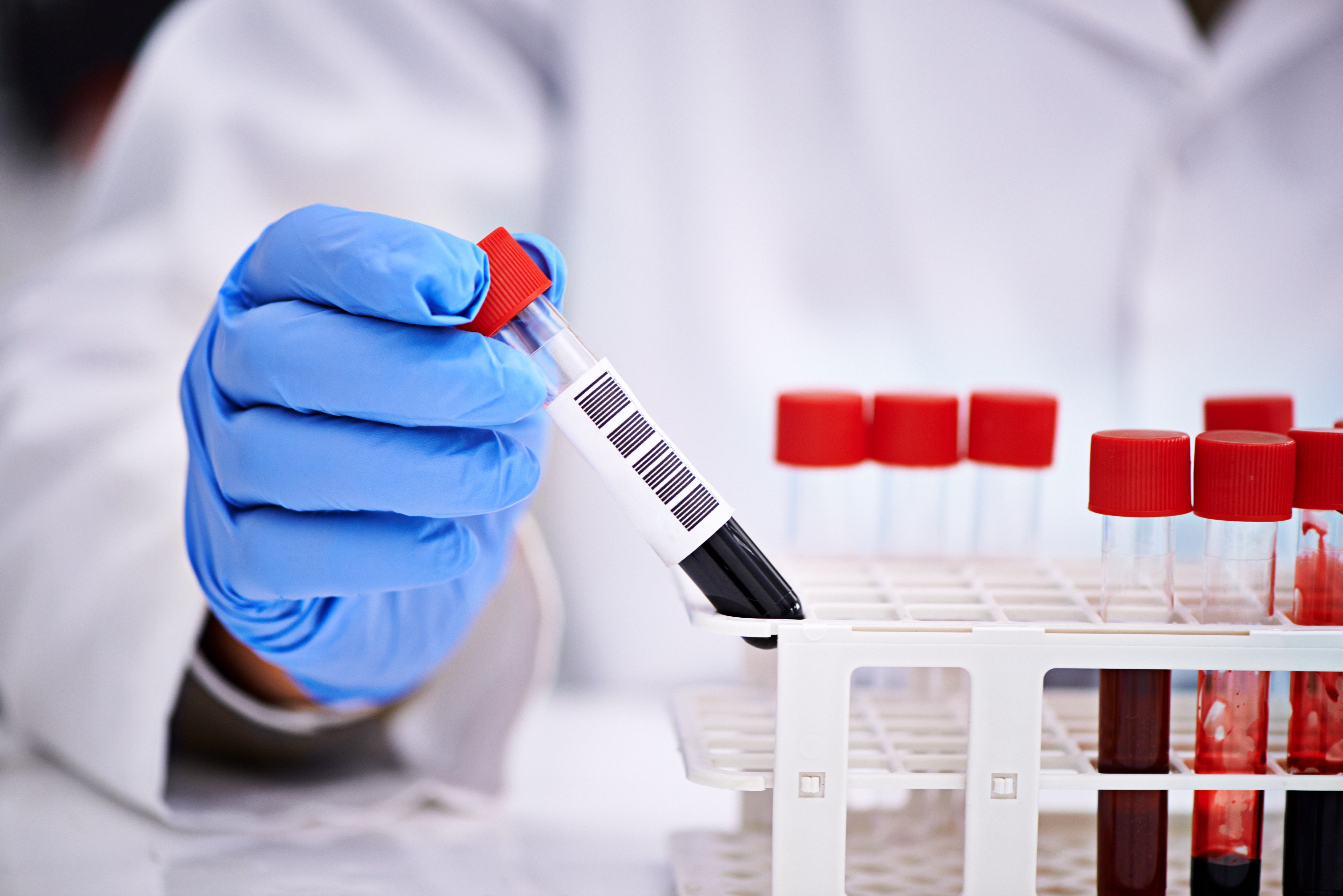
Sample Preparation to Enable Accurate Aliquoting
To ensure the samples are prepared and in a state to be isolated, they need to be introduced to the system, centrifuged to separate the plasma from the buffy coat and red blood cells, decapped to allow access, and the fraction call (or level of plasma) needs to be determined for the aliquoting process. If the samples are not prepared correctly, it could lead to incorrect or even missing results for what may be a critical blood test.
Samples are loaded to the system via pucks that allow multiple blood samples to be processed per patient and can be designed to accommodate the autonomy required for the customer. With this loading process, the patient ID is acquired and verified with the customer Lab Inventory Management System (LIMS) ensuring the sample is expected to be run that shift, otherwise offloading the sample to a default drawer for manual intervention. The criticality of the samples and difficulty in acquiring more of them guides the design of the system to allow operator intervention, ensuring the samples are not compromised while allowing additional samples to continue processing.
Physical separation of the blood sample comes next; using an automated centrifuge with customizable deceleration profiles allows samples to be spun at the required high relative centrifugal force (RCF) without remixing the samples.
Once spun the tubes move to the decapping and fraction determination process, using a vision camera to measure the top level of plasma and level of the buffy coat. The resolution of the camera and vision system lighting allows the liquid levels to be inspected accurately, with the data being recorded and used at the proceeding aliquot process. Repeated here is a default drawer, allowing any samples that don’t meet customer minimums or are compromised from a process failure to be offloaded safely for manual intervention.

High Volume Aliquoting with a BioDot Dispensing Solution
The most complex and crucial step in the plasma isolation process is the aliquoting, the process of aspirating plasma from the original patient sample and dispensing into a temporary or final container. The accuracy and efficiency of this process can be the tipping point between having enough plasma to run a trustworthy test or forcing the doctor to ask the patient for more blood draws which can be a burden depending on the patient’s situation.
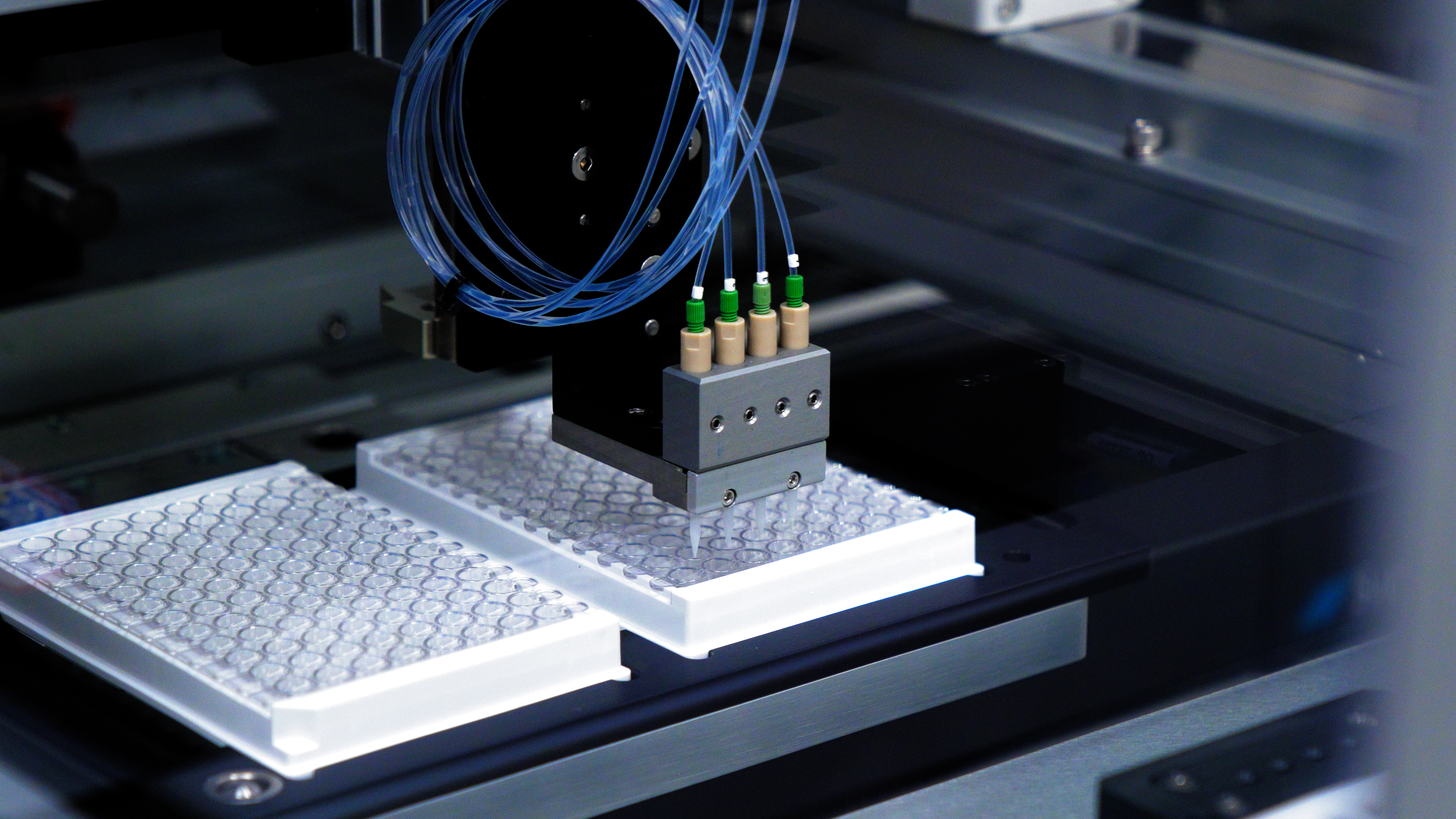
ATS’s solution achieves this by utilizing BioDot’s dispensing expertise and their newly developed 5mL aliquoting channels. Each channel is capable of aspirating up to 5mL of plasma in a single draw (compare this to almost every other automated plasma isolation system that reaches volumes only up to 1mL per draw) and can aliquot with a reported accuracy of over 95% and a CV of less than 1%. This is enabled by the high-resolution syringe pump used to draw the liquid and the tuned servo profile that tracks the plasma level as it is aliquoted. Each channel also uses a built-in pressure sensor that detects when the 5mL pipette tip reaches the top surface of the plasma which produces a smooth draw without bubbles or disruptions.
The BioDot system just requires input for the starting and stopping position for aliquoting and then takes care of the rest. The system matches servo profile to the pump speed to track the plasma level and actively monitors the pressure sensor reading to detect clogs or other complications.
While the BioDot system takes care of the aliquoting, the rest of the aliquot process is prepped by the ATS system. Used pipette tips are stripped off the units and dropped into a chute preventing any cross contamination with the samples or the machine itself. When grabbing new pipette tips the system holds up to two hours’ worth of consumable tips to allow a significant walk away time from the aliquoting process, with the benefit of also being able to refill the pipette tip storage while the machine is operating.
After aliquoting once, the process repeats by centrifuging the tubes just loaded with separated plasma, and aliquoting again to yet a third set of tubes. This repetition provides higher quality plasma leading to more accurate and reliable tests for the patients.
System Control – Designing a Robust Chain of Custody
Each patient’s data is unique and, as samples fly through our system at 500 parts per hour, it’s imperative to ensure the test results at the end of the process are matched with the correct patient. Our system manages this by effectively tracking sample identification data and processing data throughout each step of the isolation process using the built-in Programmable Logic Controller (PLC), which holds over 160 sets of patient sample data across more than 1000 individual samples in the system at any moment. This data is delivered to the customer by connecting directly to the customer’s LIMS to keep a realtime record of each critical step of the process the sample stops at.
As a sample travels through our system, its ID is validated with the LIMS as soon as it is loaded to ensure the sample is ready to process and, if not, separates the incorrect samples out automatically to allow the system to continue running while an operator investigates. This same data transfer is repeated at the remaining major process stations such as centrifugation, fraction determination, aliquoting, and offload, and communicates either updates to the samples process steps or plasma volumes. The samples are offloaded to a standard SBS format rack allowing data tracking to continue with the samples while ATS’s system communicates positions and samples within the tray for later handling.

Another option for data handling is ATS’s IlluminateTM Manufacturing Intelligence and its Part Traceability Module. Part Traceability enhances patient data management by ensuring precise tracking and documentation of each sample throughout the process. It assigns unique identifiers to samples, enabling seamless integration with LIMS. This traceability ensures that patient data is accurately linked to the corresponding samples, reducing errors and improving data integrity. It facilitates real-time monitoring and reporting, and supports efficient recall processes and audit trails, ensuring transparency and accountability.
In summary
According to Intel Market Research, the plasma separation industry is forecast to grow with a CAGR of 9.9% through 2032 and, as the market scales, the automation must scale with it. This example reflects the actual results of an ATS automated clinical lab solution that delivers 500 patient samples separated, fractionated, and isolated per hour.
Combining ATS’s experience in automation with smart conveyance by SuperTrak and low volume dispensing by BioDot, demonstrated here with the plasma isolation process, allows a robust, accurate, and compact cell which provides each process with reliable designs and repeatable sequences. Customization of the system is possible to allow different tube sizes, volumes, and media required for any process that requires sample separation. With the integration of Illuminate software and ATS’s programming, the system ensures the chain of custody is upheld and accurate results are delivered to the patients who need them.
Connect with an ATS Life Sciences Systems expert to discuss your clinical laboratory automation needs.










 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube