12th Pre-Filled Syringes, Wearable & Autoinjector Drug Delivery Summit 2026
March 11-12, 2026
NH Collection München Bavaria

This specialized conference focuses on the design, development, regulation, and future of self-administration drug delivery systems—particularly pre-filled syringes (PFS), wearable injectors/on-body delivery systems, and autoinjectors. Exchange insights on the latest regulatory updates, manufacturing advancements, and industry innovations. We at ATS Life Sciences Systems will be exhibiting at this conference. As experts in automation solutions for medical devices, ranging from low- to high-speed production, supporting efficient and compliant manufacturing.
Location
NH Collection München Bavaria
Arnulfstraße 2
80335 Munich, Germany
Event Times
March 11,2026: 08.30 am – 07.30 pm
March 12, 2026: 08.30 am – 02.00 pm
BOOK A MEETING
Visit our booth and speak to our experts about how our innovative and turnkey automation solutions can help you get the edge in the market.
Symphoni™
Digital Assembly for Medical Devices
Digital assembly automation technology featuring an unmatched combination of speed, flexibility, and precision, delivering business value in a scalable, modular platform.
Enhanced servo-based motions and e-cams mean a single system can run multiple product lines 24/7 with 90% less retooling. SYMPHONI Technology’s compact design minimizes cleanroom space. Its standardization simplifies validation. Perfect for Life Sciences applications.
Benefits: High-speed, modular assembly for complex components


ATS SwiftCell™
The SwiftCell™ family of advanced standardized assembly modules is designed to meet the highest regulatory standards making us the go-to choice for leading medical devices manufacturers in the low volume sector.
Modular solution for medical device assembly and trayhandling which is adaptable, flexible, modular and cost-effective.
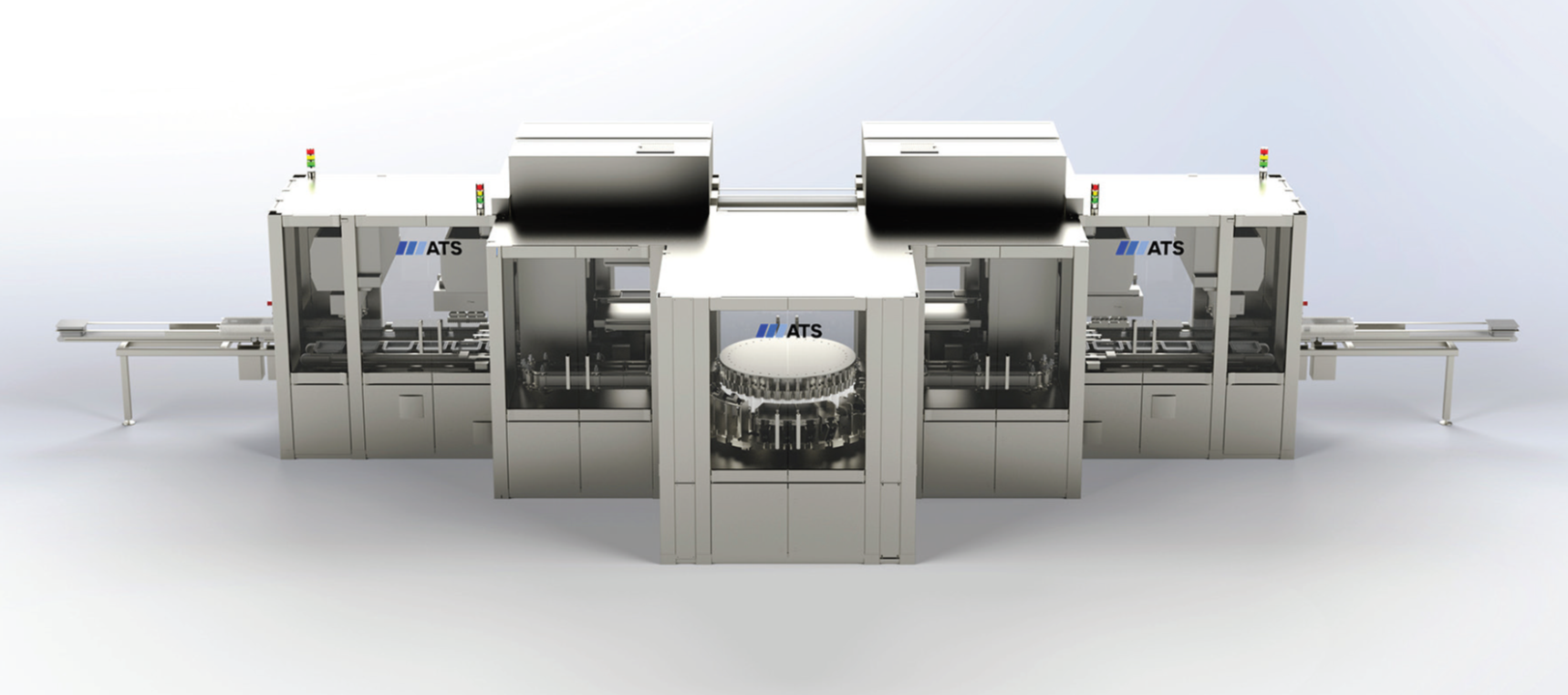
PharmaScan™ Lyo
Advanced inspection platforms for lyophilized products
This system offers near 100% accuracy at speeds of up to 425 ppm, processing approximately 60GB of images per minute. Designed for efficiency with a user-friendly interface, it handles multiple tub pitches, reducing changeovers. Built in North America and locally serviced, it’s recommended for lyophilized cakes and accommodates vials from 2R to 30R.

PharmaScan™ Liquid
Advanced inspection platforms for clear or colored liquids
This system meets FDA requirements for inspecting parenterals, handling up to 600 parts per minute with high-speed inspection that detects particles as small as 50 µm while reducing false rejects. It supports clear and colored liquids, as well as clear and colored containers, including glass and plastic, ensuring batch traceability and compliance with data handling regulations.
NH Collection München Bavaria
Munich, Germany
March 11-12, 2026


 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube